|
|
|
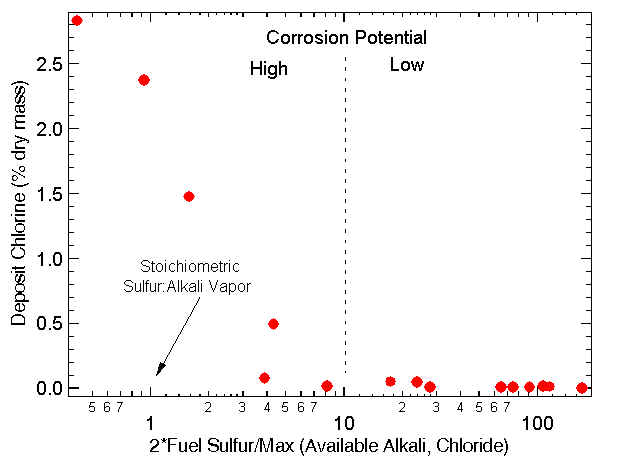
Proper management of chlorine and sulfur content can help prevent corrosion. This diagram highlights the results of pilot- and commercial-scale experiments that illustrate the effect of sulfur in helping prevent chlorine-based corrosion.
The abscissa represents a simple parameter that can be used to estimate the corrosion potential. The numerator is the total fuel sulfur and the denominator is the maximum of the available alkali or available chloride. Both are on a molar rather than mass basis. For clean biomass fuels, the available alkali and chloride are nearly as high as the total alkali and chloride. Fuels that contain additives (many forms of paper) or soil impurities (most commercial biomass) generally have significant amounts of clay-bound alkali that are not included in the available alkali.
In equilibrium, the theoretical limit of this parameter is unity. In practice, values of near ten or higher are required to prevent corrosion. This discrepancy indicates a combination of transport and kinetic limitations in the conversion of chlorides to sulfates.
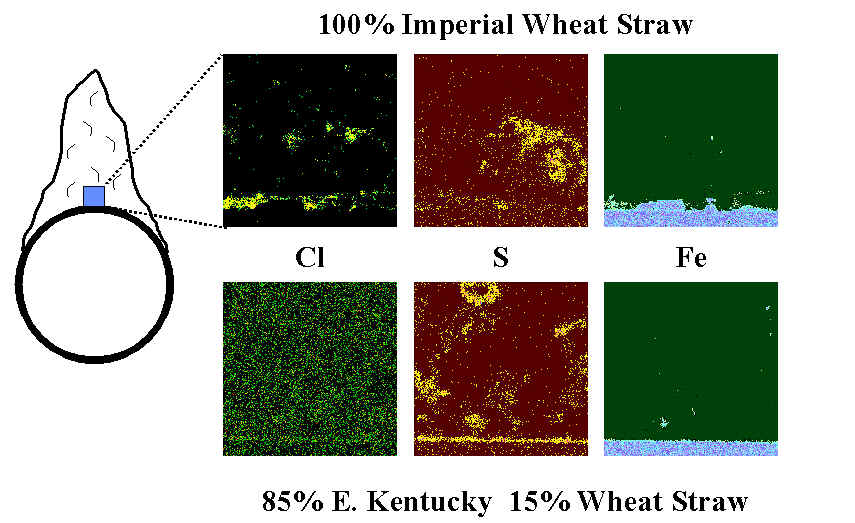
An indication of how surface chlorides lead to corrosion and how this is prevented by formation of sulfates is seen below. Here, cross sections of deposits formed when cofiring a high-chlorine biomass fuel (Imperial Wheat Straw) and a blend of this fuel with a high-rank coal are illustrated. The cross sections are taken near the leading edge of a simulated superheater tube in the MFC, and elemental maps are presented, with the deposit-tube interface near the bottom of each map. In the case of the high-chlorine fuel, a layer of chlorine is observed along the tube surface, with essentially no sulfur in the deposit (the sulfur in the micrograph is essentially background noise). The metal surface, as indicated by iron, is seen to be pitted and corroding even after a relatively short exposure. By contrast, blending the fuel with coal converts the chloride to a sulfate, as indicated by the sulfur layer along the tube surface in the results from the blend. There is no apparent corrosion of the tube under these conditions.
|
|
|
