ChEn 273 Homework Hints (Using 2019 numbering)
2.2 This problem may not have enough information supplied to get an answer. If this is the case, please state what information is missing, and make a reasonable assumption in order to answer the question.7.2
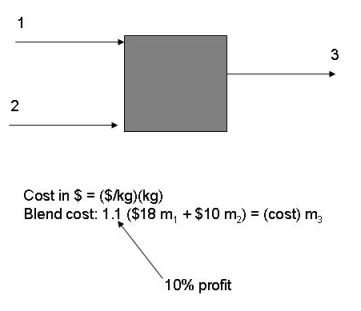
Case Study Problems
14-6. Wow- This was a hard problem. The author's answer key had lots of mistakes. The solubilities are not in the book, but are listed in Perry's Chemical Engineering Handbook. To be consistent, let's use the following solubilities:0.002 kg CaCO3/100 kg liquid H2O
0.003 kg CaSO3/100 kg liquid H2O
I found that sometimes drawing a unit separately, with the inlet and outlet streams, along with the different phases, helped me to sort through this problem. See the TA's to check answers.
On 14.6e, the second part of this problem is somewhat ambiguous. What is really wanted is the mass fraction of each component in the wet solids.
14-7. I found it a lot easier to do this problem if I calculated the heat of formation of coal. The high heating value of the coal is given, which is the negative of the heat of combustion assuming that the hydrogen goes to liquid water. All reactants and products are 25 C for this value of the heat of combustion. Since the heat of combustion is the total enthalpy of the products minus the total enthalpy of the reactants, and the total enthalpies at 25 C are merely the weighted sums of the heats of formation, the only unknown in this equation is the heat of formation of coal. Assume a basis of 100 kg of dry coal, calculate the products, and then calculate the weighted sum of enthalpies, and finally determine the heat of formation of the coal.
14-10. The only way that this problem makes sense is if the heat input to the boiler is really the heating value of the coal going into the boiler. This is the way that the regulations are written.
14-14b. Please assume that the air for combustion of the natural gas enters at 25 C.
14-14c. From the flow rate of methane, multiply the flow rate by the heat of combustion (i.e., the high heating value). This is an approximate heat addition rate. Then divide by the high heating value of the coal.