The chemical percolation devolatilization (CPD) model was developed to model
coal devolatilization based on characteristics of the chemical structure of
the parent coal. The model is intended to be an engineering model that (a) is
suitable for application in large comprehensive models of coal combustion, and
(b) accurately models key chemical structural features and reaction mechanisms
of coal. The CPD model was originally developed as a stand-alone code, but has
also been incorporated into comprehensive coal combustion models such as PCGC-3
and Fluent.
The chemical percolation devolatilization (CPD) model describes
the devolatilization behavior of rapidly heated coal based on
the chemical structure of the parent coal.[1-3] A summary document
is available that provides complete details of the development
of the CPD model.[4] The CPD model consists of five principal
components:
1. A description of the parent coal structure
2. A bridge reaction mechanism with associated kinetics
3. Percolation lattice statistics to determine the relationship between bridge breaking and detached fragments (these fragments are tar precursors)
4. A vapor-liquid equilibrium mechanism to determine the fraction of liquids that vaporize
5. A cross-linking mechanism for high molecular weight tar precursors
to reattach to the char.
Coal Structure
The CPD model is the only devolatilization model which directly uses values of measured chemical structure features. Other current models, such as Niksa's FLASCHAIN[5] and Solomon's FG-DVC[6], use a large degree of empiricism in order to get final answers that look reasonable. The CPD model uses four features of the chemical structure that are directly measured by 13-C NMR spectroscopy:
- The average molecular weight per aromatic cluster (M_cl)
- The average molecular weight per side chain (M_del)
- The average number of attachments (i.e., side chains and bridges) per cluster, referred to as the coordination number (sigma+1)
- The fraction of attachments that are bridges (P0)
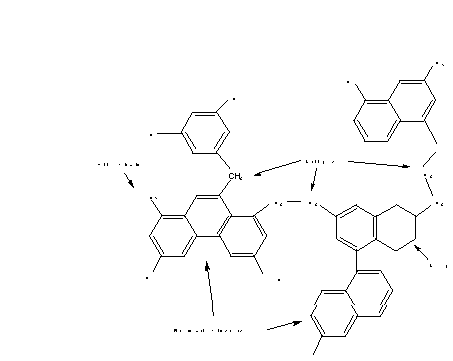
These chemical structure features are illustrated in Figure 1;
aromatic clusters are connected by bridges and loops, and side
chains serve as gas precursors. In addition, a small amount of
char bridges (C0) must be accounted for (a)
in lignites to account for early crosslinking, and (b) very high
rank coals to account for bi-aryl linkages. At present, measurements
of structural features for C0 are not available,
and an empirical relationship is used for this one variable.
Overall, direct use of 13-C NMR parameters
in the CPD model is in contrast to the previous and common practice
of adjusting input coefficients to precisely match measured tar
and total volatiles yields.
Correlation of NMR Parameters
Since the NMR parameters described above are so useful, and since they are
only available for about 30 coals, a correlation was developed to derive the
NMR-based coal structure parameters from a combination of the elemental analysis
and the proximate analysis (ASTM volatile yield). This work is described by
Genneti, Fletcher, andPugmire (Energy and Fuels, 13, 60-68, 1999) and in Genetti's
M.S. Thesis (1999).

Figure 1. Representative chemical structures identified in 13-C
NMR analyses and used in the description of coal and coal chars
in the CPD model.
Bridge Reaction Mechanism
During devolatilization, the bridges between aromatic clusters
break; aromatic clusters are thermally stable at typical devolatilization
temperatures. The bridge reaction mechanism used in the CPD model
is illustrated in Figure 2; an unreacted bridge forms a reactive
intermediate, which may either (a) cleave to form two side chains
or (b) reconnect to form a stable char bridge with release of
part of the bridge as light gas. Reaction rates for this mechanism
were obtained by comparison with measured devolatilization rates,
and are coal independent (especially at high heating rates).
Note that the gas and tar yields are a function of the chemical
structure parameters and the reaction scheme, and are therefore
not input parameters.
Percolation Lattice Statistics
Percolation lattice statistics are employed to describe the generation
of tar precursors of finite size based on the number of cleaved
labile bonds in the infinite coal lattice. This is a non-linear
relationship, and the percolation lattice statistics provide a
closed-form relationship that avoids computationally expensive
Monte Carlo technique originally proposed by Solomon.[6] A tree-like
structure called a Bethe lattice is used to approximate the coal
lattice. The Bethe lattice accounts for the crosslinking present
in the parent coal structure, as opposed to the long chain approximation
used by Niksa[5]. The Bethe lattice is fully described by the
five chemical structure parameters (Mcl, Mdel,
sigma+1, P0, and C0).
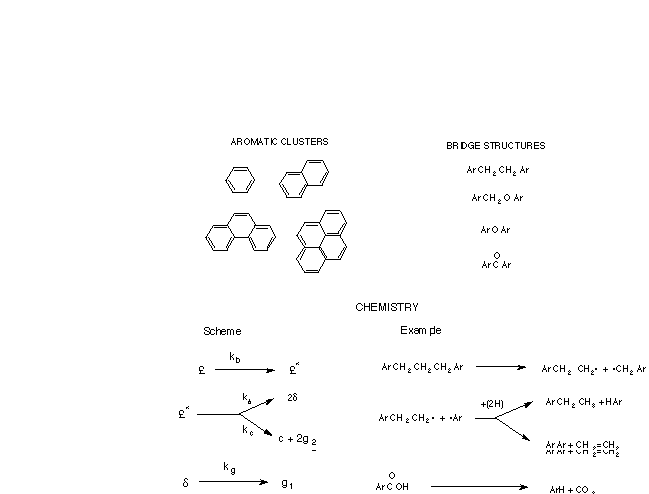
Figure 2. Representative chemical structures corresponding to
the chemical reaction scheme in the CPD model.
Vapor-Liquid Equilibrium
A generalized vapor pressure correlation for high molecular weight
hydrocarbons, such as coal tar, was developed based on data from
coal liquids. The vapor pressure of each oligomer size (monomers,
dimers, etc.) are calculated from the temperature and molecular
weight at each time step. A flash calculation is performed to
determine the fraction of each oligomer size that vaporizes at
that time step. The vapor-liquid equilibrium mechanism is principally
responsible for the change in tar yield observed as the total
pressure is changed in devolatilization experiments. The vapor
pressure relationship developed here also agrees well with pure
component vapor pressure data of 111 organic compounds thought
to exist in coal tar.[3]
Crosslinking mechanism
The crosslinking mechanism permits reattachment of metaplast (i.e.,
detached finite fragments) to the infinite char matrix. Since
details of the crosslinking mechanism are poorly known at present,
a simple empirical crosslinking rate is employed that is first
order in the amount of metaplast (detached fragments) associated
with the char. The coal-independent crosslinking rate was determined
by comparison with several sets of data.[3]
Model Performance
The CPD model successfully predicts the effects of pressure on
tar and total volatiles yields observed in heated grid experiments
for both bituminous coal and for lignite. Predictions of the
amount and characteristics of gas and tar from many different
coals compare well with available data, as shown in Figure 3,
which is unique because the majority of model input coefficients
are taken directly from NMR data, rather than used as empirical
fitting coefficients. Predicted tar molecular weights are consistent
with size-exclusion chromatography (SEC) data and field ionization
mass spectrometry (FIMS) data. Predictions of average molecular
weights of aromatic clusters as a function of coal type agree
with corresponding data from NMR analyses of parent coals.
Figure 3. Predicted and measured tar and total volatiles yields
for a wide range of coals. Perfect agreement is illustrated by
the 45 degree line. (See [3]).
The direct use of chemical structure data as a function of coal
type helps justify the model on a mechanistic rather than an empirical
basis. Empirical correlations of input parameters are available,
but these correlations tend to smooth out the features of problem
coals that can be identified with techniques such as 13-C
NMR spectroscopy. The direct measurement of chemical structural
features, along with the use of the measured features rather than
correlations, seems to be the best approach to identify specific
behaviors of coals for industry.
Since the model accurately treats the chemical features of the
coal, nitrogen release models developed from this foundation will
have a more fundamental basis and hence have the potential to
be more generally applicable than empirically-based models. The
nitrogen released during devolatilization has a major impact on
NOx reduction strategies, and is very coal
dependent. Therefore, as more is learned about the chemical forms
of nitrogen in coal, tar, and char, the CPD model provides an
ideal tool for testing new theories regarding nitrogen release.
Since the CPD model is already incorporated into comprehensive
boiler simulation codes, the new information from this contract
can be easily used by industry.
In addition to coal pyrolysis, the foundation of chemical structure
and reaction mechanisms in the CPD model have potential for application
to modeling liquefaction processes. Many of the key features
of liquefaction have parallel components in coal pyrolysis, and
since the chemical structure is correctly modeled, the transition
to liquefaction modeling is seen as promising.
The CPD model was developed in FORTRAN on a VAX system, but has
been demonstrated to work on Unix-based HP workstation and Convex
computer environments. Typical calculations are performed in
less than 1 CPU second on an HP, and the code performs well on
a personal computer.
Two versions of the CPD model are available: (1) particle temperature
versus particle residence time are required input; and (2) gas
temperature and particle residence time are required input. Version
(2) solves the particle energy equation and calls the CPD model
as a subroutine. This version includes effects of convective
heating, simple radiative heat exchange with the walls, and effects
of high mass transfer.
The CPD model has been distributed internationally, and has been
included into two publicly available comprehensive boiler simulation
codes, including:
| Richard Buckius | Univ. of Illinois |
| M. Pourkashanian | Univ. of Leeds, England |
| Woody Fiveland | Babcock & Wilcox |
| Michael Groenhout | Airflow Sciences |
| Lasse Sorensen | RISO National Laboratory, Denmark |
| Terry Wall | University of Newcastle, Australia |
and in PCGC-3 (Pulverized Coal Combustion and Gasification - 3-Dimensional;
developed at Brigham Young University) and Fluent (the largest commercial code
for 3-D computational fluid dynamics in the world).
The CPD model was developed by Thomas H. Fletcher and Alan R.
Kerstein at the Combustion Research Facility, Sandia National
Laboratories, Livermore, California 94551-0969, and Ronald J.
Pugmire, Mark Solum, and David M. Grant, Departments of Fuels
Engineering and Chemistry, University of Utah, Salt Lake City,
Utah 84112, with some follow-on work performed by Dr. Fletcher
where he is currently with the BYU Department of Chemical Engineering.
The research at Sandia was supported by the Department of Energy's
Pittsburgh Energy Technology Center's Direct Utilization AR&TD;Program
and the DOE Division of Engineering and Geosciences through the
Office of Basic Energy Sciences. The research at the University
of Utah was supported by the National Science Foundation through
the Advanced Combustion Engineering Research Center (ACERC) at
Brigham Young University and the University of Utah, by the Department
of Energy Division of Chemical Sciences, Office of Basic Energy
Sciences, and by the Associated Western Universities (AWU) who
provided summer faculty fellowships for Professors Pugmire and
Grant to spend time at Sandia. Funds for the ACERC center are
also received from the State of Utah, 75 industrial participants,
and the U.S. Department of Energy.
References
1. Grant, D. M., R. J. Pugmire, T. H. Fletcher, and A. R. Kerstein, "A Chemical Model of Coal Devolatilization Using Percolation Lattice Statistics," Energy and Fuels, 3, 175 (1989).
2. Fletcher, T. H., A. R. Kerstein, R. J. Pugmire, and D. M. Grant, "Chemical Percolation Model for Devolatilization: II. Temperature and Heating Rate Effects on Product Yields," Energy and Fuels, 4, 54 (1990).
3. Fletcher, T. H., A. R. Kerstein, R. J. Pugmire, M. S. Solum, and D. M. Grant, " A Chemical Percolation Model for Devolatilization: 3. Chemical Structure as a Function of Coal Type," Energy and Fuels, 6, 414 (1992).
4. Fletcher, T. H., A. R. Kerstein, R. J. Pugmire, M. S. Solum, and D. M. Grant, "A Chemical Percolation Model for Devolatilization: Milestone Report," Sandia report SAND92-8207, available NTIS (May, 1992).
5. Niksa, S. and A. R. Kerstein, "FLASHCHAIN Theory for Coal Devolatilization Kinetics. 1. Formulation," Energy and Fuels, 5, 647 (1991).
6. Solomon, P. R., D. G. Hamblen, R. M. Carangelo, M. A. Serio,
and G. V. Deshpande, "General Model of Coal Devolatilization,"
Energy and Fuels, 2, 405 (1988).
Dr. Fletcher home page
, ACERC home page
, BYU ChE Dept home page
, BYU home page