|
|
|
The introduction of chlorine-laden fuels into combustors has the potential to introduce chlorine-based corrosion problems. At combustion temperatures, the most thermodynamically preferred chlorine-containing species are alkali chlorides and hydrochloric acid (in that order). The most preferred forms of alkali are alkali chlorides and alkali hydroxides (again in that order). At the temperatures of heat transfer surfaces and deposits, alkali sulfates are more stable than the chlorides. The tendency in the combustor is for the high-temperature chlorides to condense or otherwise deposit on heat transfer surfaces and form sulfates. If there is sufficient sulfur and time available, this conversion will significantly retard corrosion of the heat transfer surface. However, if there is insufficient sulfur to convert the chlorides to sulfides (as is the case with many pure biomass fuels), rapid corrosion of superheaters and other high-temperature surfaces may result.
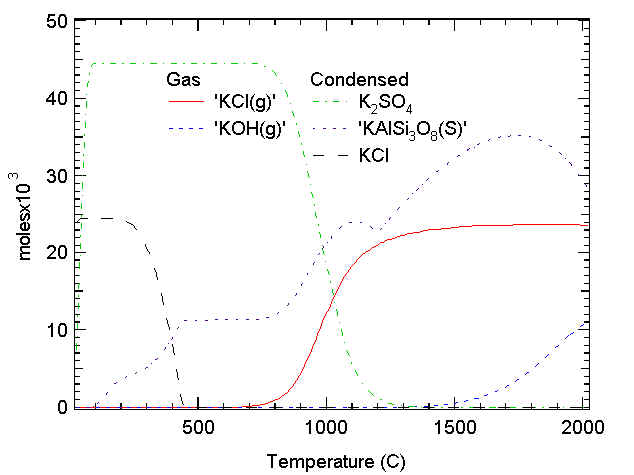
The results from this thermochemical equilibrium calculation illustrate some of the major alkali- and chlorine-containing species formed at various temperatures under combustion conditions. The full mixture of species is quite complex, but these trends illustrate how chlorides in the gas phase form sulfates in the condensed phase as temperatures drop from combustion temperatures to surface temperatures. In this case, there are fewer sulfur atoms than chlorine or silica atoms. In the absence of sulfur, condensed KCl would be present at all superheater temperatures.
|
|
|
