
Homework
Homework Hints
- 3.59 In part (a) derive expressions for a fractional linear density in terms of R radius of the atom, and in part (b) calculate the atomic linear densities for Fe in each direction.
- 3.61 In part (a) derive expressions for a fractional planar density, and in part (b) calculate an atomic planar density for Mo in these 2 planes.
- 3.73 Use peak angles of 2 as 40.2, 58.4, 73.3, 87.0 and 100.7. The lowest indices produce the lowest angle and strongest peak.
- 4.6 More than one choice is possible for parts (b) and (c), so give at least 2 examples. Explain your reasoning for each choice.
- 4.42 In this problem, surface energy refers to the outer surface of a chunk of metal, whereas the grain boundary that they want you to consider is the energy at a grain boundary inside the polycrystalline metal.
- 7.6 Do part (a) in units of atomic planar density and part (b) in fractional planar density.
- 14.5 Part c: use a number average degree of polymerization (equation 14.6).
- 16.7 Start the calculation for the plot in part (a) at l = 3 mm and go to l = 50 mm.
- 17.11 Ignore the hokey equation 17.23 and solve this from first principles and unit conversions.
- 17.19 On Fig 17.27, there is no line drawn yet for the cathodic reaction. So to help you, draw a straight line representing the cathodic reaction originating near the V of Velocity and slopes upward at about a 30 angle and intersects all of the solution velocity curves, as shown below.
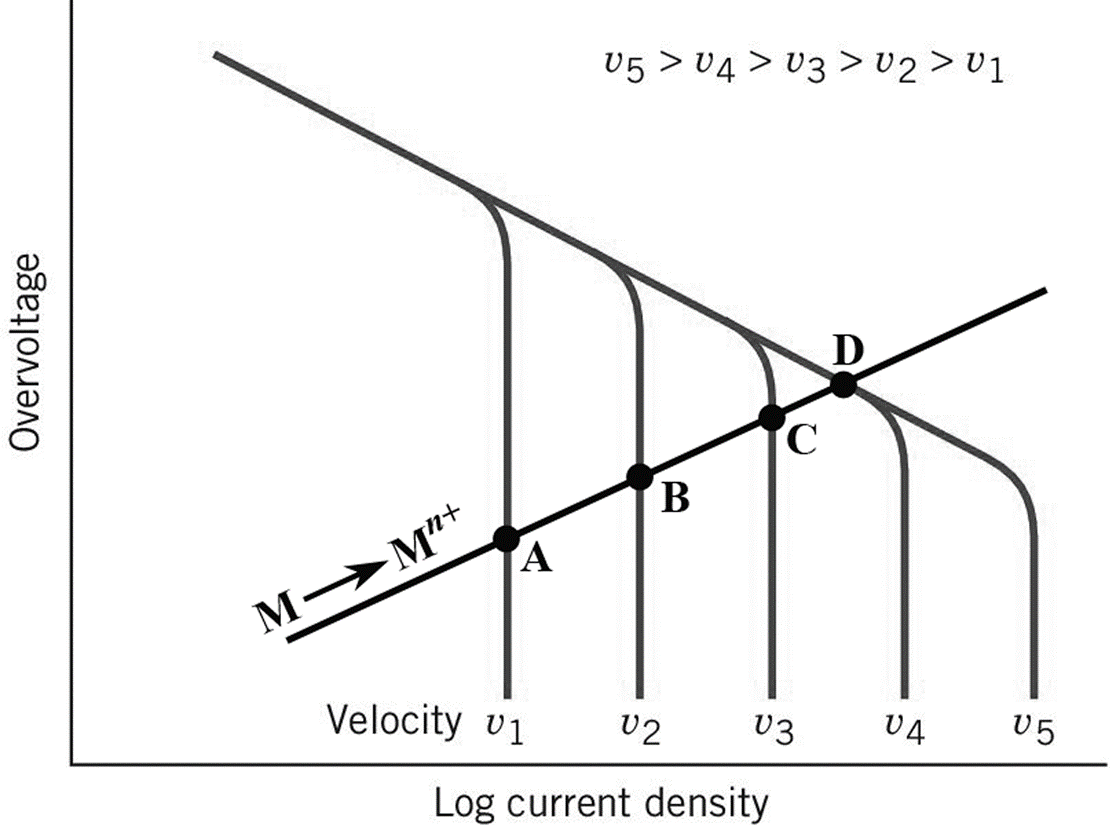
- 17.24 Look at Fig 17.24 for an example of a large anode-to-cathode situation, and reverse the picture for a small anode-to-cathode situation. What is the cathodic reaction? Is there any concentration polarization on the cathodic reaction? So what limits electron flow and the resulting corrosion?

